Abstract
Introduction: Accumulating evidence indicates that prostaglandin D2 synthase (PTGDS) plays important roles in various diseases, including tumor. Ferroptosis, a recently recognized form of cell death, has been found to participate in the development of hematologic tumor, especially lymphoma. Here, we investigated the effects of AT56, the specific inhibitor of PTGDS, and its regulatory role on ferroptosis in peripheral T cell lymphoma (PTCL).
Methods: Lymph node biopsies from 112 de novo PTCL patients and 38 reactive hyperplasia cases. RNA-sequencing and Tandem mass tag (TMT)-mass spectrometry were conducted in PTCL cells treated with AT56. C11-BODIPY was used to detect the level of lipid ROS. Xenograft PTCL mice model was established to evaluate drug efficacy. Co-immunoprecipitation (Co-IP) and confocal immunofluorescence (IF) were performed to validate protein interaction. Plasmids of HMOX1-WT and HMOX1-H25A were synthesized and transfected into PTCL cells.
Results: Firstly, we explored the expression and function of PTGDS in PTCL. The expression level of PTGDS in tumor tissue (n=112) was found to be higher than control group. Survival analysis revealed that elevated PTGDS expression predicted inferior prognosis in PTCL patients. Gene ontology (GO) analysis indicated that AT56 treatment was associated with biological processes involved in tumor progression, such as cell death, apoptosis, and cell cycle. Then, AT56 could inhibit the proliferation of PTCL cells in a dose- and time-dependent manner.
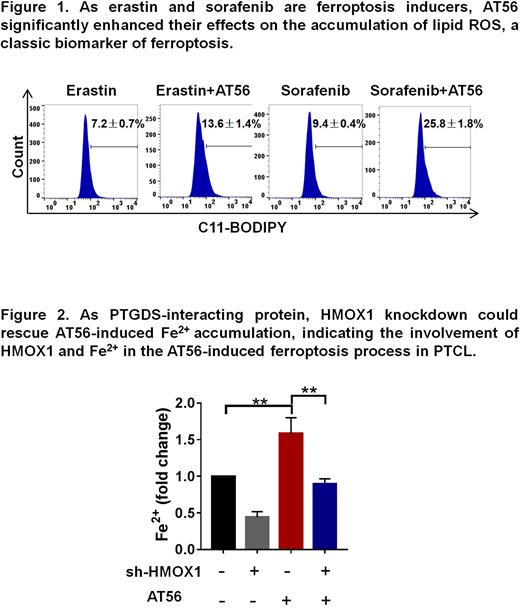
Analysis based on TMT-mass spectrometry revealed the differential expression of ferroptosis-associated proteins after AT56 treatment. As erastin and sorafenib are ferroptosis inducers, AT56 significantly enhanced their effects on the accumulation of lipid ROS (Fig. 1), a classic biomarker of ferroptosis. Besides, ferrostatin-1(Fer-1), a specific ferroptosis inhibitor reversed AT56-induced lipid ROS accumulation and cell proliferation inhibition. Furthermore, the combined drug effects of AT56 and sorafenib was revealed in xenograft mice model, indicating the regulatory role of AT56 on ferroptosis in PTCL.
As ferroptosis is characterized by its dependence on iron accumulation, reactive oxygen species (ROS) production, lipid peroxidation, we further explored the specific mechanism by which AT56 regulated ferroptosis process. In our study, ferroptosis-associated molecules (NRF2, KEAP1) and iron-associated molecules (NCOA4, FTH, FTL) were aberrantly expressed after AT56 treatment, indicating ferroptosis activation, while the expression of lipid and ROS-associated molecules (ACSL4, xCT, GPX4) displayed compensatory changes. As expected, deferoxamine (DFO), an iron chelator, rescued the effect of AT56 on cell proliferation and lipid ROS accumulation. Elevated level of Fe2+ was found in tumor tissue from mice receiving AT56, indicating that the role of AT56 on ferroptosis mainly depended on iron accumulation.
High expression of NCOA4 indicated the involvement of autophagy in ferroptosis process induced by AT56. Chloroquine, a common autophagy inhibitor, could partly reverse AT56-induced accumulation of lipid ROS and Fe2+, and expression of Fe2+ storage protein, suggesting the dependence of AT56-induced ferroptosis on autophagy in PTCL cells.
Structure analysis based on TMT-mass spectrometry identified HMOX1, a regulator of heme catabolism and autophagy, as PTGDS-interacting protein, and further Co-IP and IF assays validated their co-localization and interaction in PTCL cells. Besides, HMOX1 knockdown could rescue AT56-induced cell proliferation inhibition and Fe2+ accumulation (Fig. 2). Furthermore, point mutation of HMOX1 (H25A) was found to inhibit its regulatory role on ferroptosis.
Conclusions: Taken together, our investigations identified for the first time that high expression of PTGDS was associated with poor prognosis in PTCL patients. AT56 exerted potent anti-tumor effects in PTCL via promoting ferroptosis process, which was mediated by HMOX1 and partly dependent on autophagy. Our in vitro and in vivo study revealed the synergistic effect and mechanism of AT56 and sorafenib, providing a novel AT56-based treatment paradigm for PTCL patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal